Polymer Micelle Structures July 30, 2009
Posted by desporatist in Polymer Micelles as Drug Carriers.trackback
Self-assembled micelles
Self-assembled polymer micelles are created from amphiphilic polymers that spontaneously form nanosized aggregates when the individual polymer chains (“unimers”) are directly dissolved in aqueous solution (dissolution method)above a threshold concentration (critical micelle concentration or CMC) and solution temperature (critical micelle temperature or CMT) (Fig. 1). Amphiphilic polymers with very low water solubility can alternatively be dissolved in a volatile organic solvent, then dialyzed against an aqueous buffer (dialysis method).
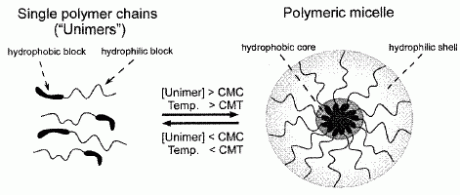
Fig. 1. Self-assembly of block copolymer micelles.
Amphiphilic diblock (hydrophilichydrophobic) or triblock (hydrophilichydrophobichydrophilic) copolymers are most commonly used to prepare selfassembled polymer micelles for drug delivery, although the use of graft copolymers has been reported. For drug delivery purposes, the individual unimers are designed to be biodegradable and/or have a low enough molecular mass (< ~40 kDa) to be eliminated by renal clearance, in order to avoid polymer buildup within the body that can potentially lead to toxicity. The most developed amphiphilic block copolymers assemble into spherical coreshell micelles approximately 10 to 80 nm in diameter, consisting of a hydrophobic core for drug loading and a hydrophilic shell that acts as a physical (“steric”) barrier to both micelle aggregation in solution, and to protein binding and opsonization during systemic administration (Fig. 2). The most common hydrophilic block used to form the hydrophilic shell is the FDAapproved excipient poly(ethylene glycol) (PEG) or poly(ethylene oxide) (PEO). PEG or PEO consists of the same repeating monomer subunit CH2CH2O, and may have different terminal end groups, depending on the synthesis procedure, e.g. hydroxyl group HO(CH2CH20)nH; methoxy group CH30(CH2CH20)nH, etc. PEG/PEO blocks typically range from 1 to 15 kDa.In addition to its FDA approval, PEG is extremely soluble and has a large excluded volume. This makes it especially suitable for physically interfering with intramicelle interactions and subsequent micelle aggregation. PEG also blocks protein and cell surface interactions, which greatly decreases nanoparticle uptake by the reticuloendothelial system (RES), and consequently increases the plasma half life of the polymer micelle. The degree of steric protection by the hydrophilicshell is a function of both the density and length of the hydrophilic PEG blocks.
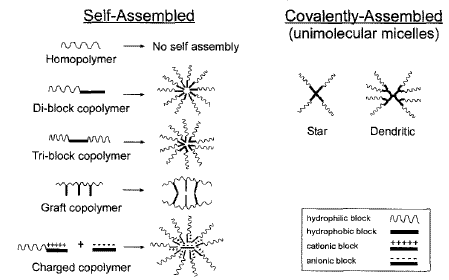
Fig. 2. Polymer micelle structures.
Unlike the hydrophilic block, which is typically PEG or PEO, different types of hydrophobic blocks have been sufficiently developed as hydrophobic drug loading cores. Examples of diblock copolymers include (a) poly(Lamino acids), (b) biodegradable poly(esters), which includes poly(glycolic acid), poly(D lactic acid), poly(D,Llactic acid), copolymers of lactide/glycolide, and poly(ecaprolactone), (c) phospholipids/long chain fatty acids; and for triblock copolymers, (d) polypropylene oxide (in Pluronics/poloxamers). The choice of hydrophobic block is largely dictated by drug compatibility with the hydrophobic core (when drug is physically loaded, as described later) and the kinetic stability of the micelle. The selfassembly of amphiphilic copolymers is a thermodynamic and, consequently, a reversible process that is entropically driven by the release of ordered water from hydrophobic blocks; it is either stabilized or destabilized by solvent interactions with the hydrophilic shell. As such, the structural potential of amphiphilic copolymer unimers to form micelles is determined by the mass ratio of hydrophilic to hydrophobic blocks, which also affects the subsequent morphology if aggregates are formed. If the mass of the hydrophilic block is too great, the copolymers exist in aqueous solution as unimers, whereas, if the mass of the hydrophobic block is too great, unimer aggregates with nonmicellar morphology are formed. If the mass of the hydrophilic block is similar or slightly greater than the hydrophobic block, then conventional core shell micelles are formed. An important consideration for drug delivery is the relative thermodynamic (potential for disassembly) and kinetic (rate of disassembly) stability of the polymer micelle complexes, after intravenous injection and subsequent extreme dilution in the vascular compartment. This is because the polymer micelles must be stable enough to avoid burst release of the drug cargo, as in the case of a physically loaded drug, upon systemic administration and remain as nanoparticles long enough to accumulate in sufficient concentrations at the target site. The relative thermodynamic stability of polymer micelles (which is inversely related to the CMC) is primarily controled by the length of the hydrophobic block.An increase in the length of the hydrophobic block alone significantly decreases the CMC of the unimer construct (i.e. increases the thermodynamic stability of the polymer micelle), whereas an increase in the hydrophilic block alone slightly increases the CMC (i.e. decrease the thermodynamic stability of a polymer micelle).Although the CMC indicates the unimer concentration below which polymer micelles will begin to disassemble, the kinetic stability determines the rate at which polymer micelle disassembly occurs. Many diblock copolymer micelles possess good kinetic stability and only slowly dissociate into unimers after extreme dilution. Thus, although polymer micelles are diluted well below typical unimer CMCs (10~6107M) after intravenous injection, their relative kinetic stability might still be suitable for drug delivery. The kinetic stability depends on several factors, including the size of a hydrophobic block, the mass ratio of hydrophilic to hydrophobic blocks, and the physical state of the micelle core. The incorporation of hydrophobic drugs may also further enhance micelle stability.
Unimolecular micelles
Unimolecular micelles are topologically similar to selfassembled micelles, but consist of single polymer molecules with covalently linked amphiphile chains. For example, copolymers with starlike or dendritic architecture, depending on their structure and composition, can either aggregate into multimolecular micelles,or exist as unimolecular micelles. Dendrimers are widely used as building blocks to prepare unimolecular micelles, because they are highlybranched, have welldefined globular shape and controled surface functionality. For example, unimolecular micelles were prepared by coupling dendritic hypercores of different generations with PEO chains. The dendritic cores can entrap various drug molecules. However, due to the structural limitations involved in the synthesis of dendrimers of higher generation, and relatively compact structure of the dendrimers, the loading capacity of such micelles is limited. Thus, to increase the loading capacity, the dendrimer core can be modified with hydrophobic block, followed by the attachment of the PEO chains. For example, Wang et al. recently synthesized an amphiphilic 16arm star polymer with a polyamidoamine dendrimer core and arms composed of inner lipophilic poly(ecaprolactone) block and outer PEO block. These unimolecular micelles were shown to encapsulate a hydrophobic drug, etoposide, with high loading capacity. Multiarm starlike block copolymers represent another type of unimolecular micelles. Star polymers are generally synthesized by either the armfirst or corefirst methods. In the armfirst method, monofunctional living linear macromolecules are synthesized and then crosslinked either through propagation, using a bifunctional comonomer, or by adding a multifunctional terminating agent to connect precise number of arms to one center. Conversely, in the corefirst method, polymer chains are grown from a multifunctional initiator. One of the first reported examples of unimolecular micelles, suitable for drug delivery, was a threearm star polymer, composed of mucic acid substituted with fatty acids as a lipophilic inner block and PEO as a hydrophilic outer block. These polymers were directly dispersible in aqueous solutions and formed unimolecular micelles. The size and solubilizing capacity of the micelles were varied by changing the ratio of the hydrophilic and lipophilic moieties. In addition, starcopolymers with polyelectrolyte arms can be prepared to develop pHsensitive unimolecular micelles as drug carriers.
Cross-linked micelles
The multimolecular micelles structure can be reinforced by the formation of crosslinks between the polymer chains. These resulting crosslinked micelles are, in essence, single molecules of nanoscale size that are stabile upon dilution, shear forces and environmental variations (e.g. changes in pH, ionic strength, solvents etc.). There are several reports on the stabilization of the polymer micelles by crosslinking either within the core domain or throughout the shell layer. In these cases, the crosslinked micelles maintained small size and coreshell morphology, while their dissociation was permanently suppressed. Stable nanospheres from the PEObpolylactide micelles were prepared by using polymerizable group at the core segment. In addition to stabilization, the core polymerized micelles readily solubilized rather large molecules such as paclitaxel, and retained high loading capacity even upon dilution. Formation of interpenetrating network of a temperaturesensitive polymer (polyNisopropylacrilomide) inside the core was also employed for the stabilization of the Pluronic micelles. The resulting micelle structures were stable against dilution, exhibited temperatureresponsive swelling behavior, and showed higher drug loading capacity than regular Pluronic micelles. Recently, a novel type of polymer micelles with crosslinked ionic cores was prepared by using block ionomer complexes as templates. The nanofabrication of these micelles involved condensation of PEObpoly(sodium methacrylate) diblock copolymers by divalent metal cations into spherical micelles of coreshell morphology. The core of the micelle was further chemically crosslinked and cations removed by dialysis. Resulting micelles represent hydrophilic nanospheres of coreshell morphology. The core comprises a network of the crosslinked polyanions and can encapsulate oppositely charged therapeutic and diagnostic agents, while a hydrophilic PEO shell provides for increased solubility. Furthermore, these micelles displayed the pH and ionic strengthresponsive hydrogellike behavior, due to the effect of the crosslinked ionic core. Such behavior is instrumental for the design of drug carriers with controled loading and release characteristics.
Comments»
No comments yet — be the first.